Introduction.The fibrinolytic system is crucial for clot lysis during wound healing. Clot lysis is initiated by tissue plasminogen activator (tPA) and urokinase plasminogen activator (uPA), which convert plasminogen into plasmin. TPA-mediated plasminogen activation requires binding to fibrin, while uPA activates plasminogen independent of fibrin. Both plasminogen activators are inhibited by plasminogen activator inhibitor-1 (PAI-1), although, fibrin-bound tPA is protected from PAI-1. As inadequate regulation of fibrinolysis might lead to thrombotic and bleeding events, there is a clinical need for tools that estimate a patient's fibrinolytic state. We developed anti-fibrin-uPA (aFn-uPA), a construct containing the plasminogen activator uPA, linked to a nanobody directed against fibrin, to support binding of uPA to fibrin, and to increase effectiveness of plasminogen activation. This novel plasminogen activator improves the sensitivity of the calibrated, automated plasmin generation (PG) assay, for PAI-1.
Aim.To increase the sensitivity of the calibrated, automated PG assay for detection of (anti-)PAI-1 effects in human plasma.
Methods.PG in normal pooled plasma (NPP) or factor deficient plasma was measured by cleavage of a plasmin-specific fluorogenic substrate, using a calibrated, automated method. In brief, fibrinolysis was started by combining plasma and trigger solution in a 96-well plate. The trigger solution consists of tissue factor (TF) or Russell's viper venom-factor X activator (RVV-X), phospholipids, and tPA or aFn-uPA. PG was measured with a fluorometer, equipped with a 390/460 filter set (excitation/emission) (Fluoroscan Ascent, Thrombinoscope, Maastricht, The Netherlands). The following PG parameters were extracted from the data: lag time, time to peak (ttPeak), endogenous plasmin potential (EPP) and peak.
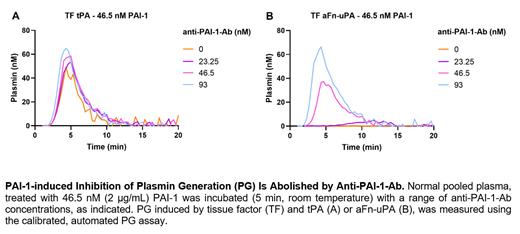
Results.High TF, RVV-X, tPA and aFn-uPA were associated with a shorter PG lag time and a shorter PG ttPeak, compared to lower trigger concentrations. For subsequent experiments, 5 pM TF or 10 -5 RVV-X, and 1.25 µg/mL (17.8 nM) tPA or 1 µg/mL (21 nM) aFn-uPA, were selected as optimal. The assay was specific for plasmin as no PG was observed in plasminogen- nor fibrinogen-deficient plasma. The sensitivity of the PG assay to PAI-1 was higher in PG triggered with the novel plasminogen activator aFn-uPA, compared to tPA, regardless of the affinity of both activators for fibrin. PAI-1 dose-dependently increased the lag time and ttPeak, and reduced the EPP and peak in PG stimulated with aFn-uPA in both NPP and PAI-1-deficient plasma. Using an anti-PAI-1-Ab, the inhibition obtained upon 2 µg/mL (46.5 nM) PAI-1, was reduced or completely abolished, depending on the concentration of anti-PAI-1-Ab (Figure).
Conclusions.Overall, the calibrated, automated PG assay, in particular when aFn-uPa is used as trigger for fibrinolysis, is a specific and efficient tool to measure PG in human plasma, and is sensitive to the therapeutic target PAI-1. This tool may help identify abnormal fibrinolytic states in bleeding and thrombotic disorders.
Disclosures
De Simone:Diagnostica Stago: Other: Synapse Research Institute is part of the Diagnostica Stago group. Roest:Synapse Research Institute: Current Employment, Other: Synapse Research Institute is part of the Diagnostica Stago group. de Laat:Diagnostica Stago: Other: Synapse Research Institute is part of the Diagnostica Stago group. Huskens:Synapse Research Institute: Current Employment, Other: Synapse Research Institute is part of the Diagnostica Stago group.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal